Meridian Medical Technologies, a Pfizer company that makes Mylan’s EpiPen injector, issued a nationwide, voluntary recall— including the children’s version, EpiPen Jr.
“The potential defect could make the device difficult to activate in an emergency (failure to activate or increased force needed to activate) and have significant health consequences for a patient experiencing a life-threatening allergic reaction (anaphylaxis),” company says in a statement.
The company recalled the product in several countries last month following two reports of the device failing to activate. It says only one lot was found to be flawed but as precautionary measure a total of 13 lots are now being recalled from the US market.
If you think you may be impacted by this recall, please follow these steps:
STEP 1: Check the lot number on your carton or device to see if your EpiPen® Auto-Injector is affected by the recall.
STEP 2: If your EpiPen® Auto-Injector has been recalled, contact Stericycle at 877-650-3494 to obtain a voucher code for your free replacement product. Stericycle also will provide you with a pre-paid return package to ship the product back to Stericycle.
STEP 3: Visit your pharmacy with your voucher information to redeem your free replacement.
STEP 4: Send your recalled product to Stericycle. Do not return any devices affected by the recall until you have your replacement in hand.
Stericycle’s hours of operation are Monday-Friday 8 a.m.-10 p.m. ET, and Saturday and Sunday 8 a.m.-5 p.m. ET.
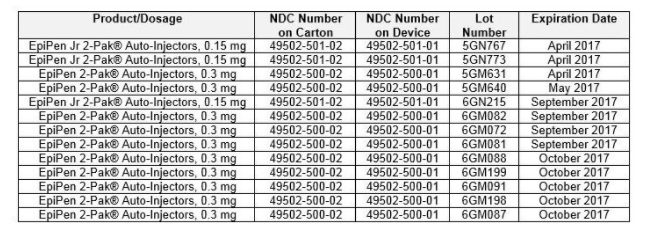
Here is the list of U.S. Impacted Lots:
- EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg – Lot number: 5GN767 – Expiration: April 2017
- EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg – Lot number: 5GN773 – Expiration: April 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 5GM631 – Expiration: April 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 5GM640 – Expiration: May 2017
- EpiPen Jr 2-Pak® Auto-Injectors, 0.15 mg – Lot number: 6GN215 – Expiration: September 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 6GM082 – Expiration: September 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 6GM072 – Expiration: September 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 6GM081 – Expiration: September 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 6GM088 – Expiration: October 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 6GM199 – Expiration: October 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 6GM091 – Expiration: October 2017
- EpiPen 2-Pak® Auto-Injectors, 0.3 mg – Lot number: 6GM198 – Expiration: October 2017
- EpiPen 2-pak® Auto-Injectors, 0.3 mg – Lot number: 6GM087 -Expiration: October 2017
From USA Today: https://www.usatoday.com/story/news/nation-now/2017/04/03/epipen-being-recalled-over-potential-defect/99977396/
From Mylan: http://www.mylan.com/en/epipenrecall